A. Learning Objectives
In this lab, students will:
• analyze the effect of catechol oxidase on the production of benzoquinone.
• design and conduct experiments to study how physical conditions affect enzyme activity.
• plot data graphically.
B. Textbook Correlation
Please review Section 6.2 Enzymes and Ribozymes in Chapter 6: An Introduction to Energy, Enzymes, and Metabolism to assist in writing the intrroduction and researching the experiment.
C. Introduction
Please write a two paragraph introduction to enzymes;
Paragraph #1: Discuss the structure/function of enzymes. In your discussion, address the make-up of most enzymes, the role of the active site and its impact on specificity, and the idea behind the induced fit theory, Also discuss activation energy and how enzymes speed up chemical reactions by impacting the activation energy.
Many cellular processes in the body are able to occur because of catalysts. The most common type of catalyst in our bodies are enzymes, which are generally proteins. When chemical reactions occur throughout the body, covalent bonds between molecules are broken and/or formed. In order to correctly undergo these chemical reaction, usually the molecules have to be oriented in a certain manner and contain a certain amount of energy in order to undergo the process. This initial input of energy is called the activation energy. But many processes do not have enough energy to go through the process themselves, they require the help of an enzyme. The enzyme will lower the activation energy and allow the molecules to reach their transition state (a state in which the original bonds are stretched to the maximum). They do this by either straining the reactants or bringing the molecules closer together. Every enzyme has an active site, which is where the substrate binds in order to create the enzyme-substrate complex. Enzymes have a high degree of specificity, which means they are extremely specific to the molecular structure of the substrate. When the substrate binds tightly to the enzyme, the enzyme will undergo conformational changes in order to better fit the substrate. This is called induced fit.
Paragraph #2: Discuss how enzyme activity is regulated in a cell. Include in the discussion the idea of enzyme saturation, how saturation is overcome, the physical requirements for optimal enzyme activity, and the role of inhibitors (both competitive and non-competitive). When discussing inhibitors, include the idea behind allosteric regulation.
One factor that regulates enzyme activity is the affinity the enzyme has to its substrate. Enzymes with high affinities to its substrate will continue to bind even when substrate concentrations are low, however, the enzyme-substrate complex is more likely to form with higher levels of substrate. Studies have shown that the velocity of the reaction increases as the substrate concentration, but it will eventually reach a plateau called the Vmax. This occurs when all the active sites of the enzyme are occupied and increasing the substrate concentration would have no effect; also known as enzyme saturation. In order to overcome this saturation you can either lower the substrate concentration or increase the enzyme concentration in order to catalyze more reactions. The rate of the reaction is also dependent on the environmental conditions: temperature, pH, and ionic conditions. Many enzymes work in a narrow range of temperature and pH, depending on the particular enzyme. Going out of this range can result in a denatured and dysfunctional enzyme. In addition to these factors, inhibitors play a major role in regulating enzyme activity. Both reversible and irreversible inhibitors exist, but reversible ones are more prevalent within the cell. These are classified as either competitive and noncompetitive. Competitive inhibitors are molecules that resemble the substrate and bind to the active site of the enzyme, preventing the substrate from binding. These inhibitors compete for the active site and in order to overcome these inhibitors, the substrate concentration is increased. Then there's non competitive inhibitors. These molecules will bind to the allosteric site of the enzyme and alter the conformational structure of the enzyme, preventing the substrate from binding. These allosteric sites can be used for enzyme inhibition or enzyme activation, by indirectly changing the active site.
| INSERT AN IMAGE SHOWING A PLOT OF ENZYME ACTIVITY VS SUBSTRATE CONCENTRATION. THIS IMAGE SHOULD DEMONSTRATE ENZYME SATURATION BY SUSBTRATES. |
INSERT AN IMAGE OR ANIMATION DEMONSTRATING THE ROLES OF COMPETITIVE VS NON-COMPETITIVE INHIBTORS |
INSERT AN IMAGE/ANIMATION SHOWING ALLOSTERIC REGULATION OF ENZYMES WITHIN A CELL |
 |
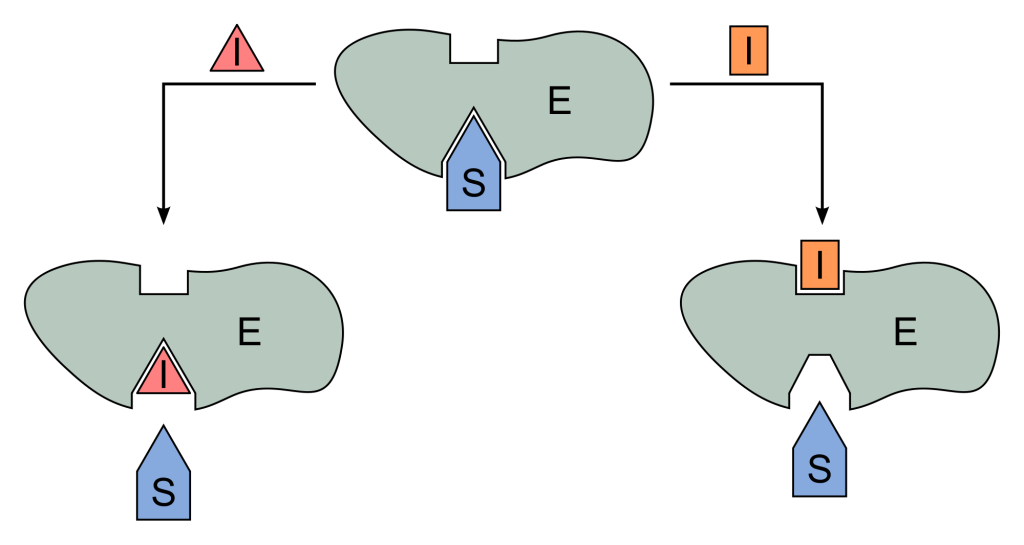 |

|
In today’s exercise you will first observe the actions of the enzyme catechol oxidase. After this exercise you will be ready to design two experiments on your own to test the physical requirements for optimal enzyme activity.
D. Catechol Oxidase Activity
In today’s exercise the enzyme you will use is catechol oxidase. In plants this copper-containing enzyme creates brown pigment when exposed to air (specifically oxygen), and it is the reason fruits turn brown after they are sliced. The brown color is due to the production of the product benzoquinone, a substance that is toxic to food-spoiling bacteria. When the peel is damaged, oxygen can then react with the catechol, protecting the fruit.
In this experiment, we will test catechol oxidase activity. The enzyme is extracted from potatoes using a blender and is referred to as potato extract in the subsequent experiments.
Experimental Procedure:
1. Label 3 test tubes 1–3.
2. Pipette the amount of catechol and water into the appropriate test tube as outlined in Table 1. Do not add the catechol oxidase to all tubes until just before starting the incubation in step 3.
|
Tube
|
mL of Catechol
|
mL of Water
|
mL of Catechol Oxidase
|
|
1
|
1
|
0
|
1 mL (20 drops)
|
|
2
|
0
|
1
|
1 mL (20 drops)
|
|
3
|
1
|
1
|
0 mL (0 drops)
|
3. Place the test tubes in the 37⁰C water bath for 10 minutes.
4. Record your results in the table above. Use the following scale:
0 no color change
1 little color change
2 more color change
3 dark color change
|
Tube
|
Result
|
Conclusion
|
|
1
|
|
|
|
2
|
|
|
|
3
|
|
|
Questions
1. Which tube is the negative control? Which tube is your positive control?
2. What would it mean if tube 2 turned brown?
E. Design an Experiment to Study Enzyme Activity Under Different Physical Conditions.
Protein activity is highly dependent on its three-dimensional structure. Conditions that cause a protein to denature (unfold) results in the loss of protein activity. Environmental deviations from optimal cause an enzyme to lose activity. Question: What is the optimal temperature for catechol oxidase activity? What is the ideal pH for catechol oxidase activity? What is the optimal salinity for catechol oxidase activity? Use the experiment from section D as a template. Remember to include positive and negative controls when applicable. Make sure you take photographic images of your results and a video of your procedure explaining how you designed the experiment.
Materials Provided:
- test tubes
- plastic pipettes
- catechol
- potato extract
- water baths (3) and hot plate
- ice
- thermometers
- phosphate buffers ranging from pH of 2 to 12 (actual buffers available (in pH units): 2, 4, 6, 7, 8, 10, 12)
- distilled water
- 10% NaCl stock solution
Experiment #1: Temperature
1. Hypothesis: The optimal temperature for catechol oxidase is 40 degrees Celsius (this is because this is close to room temperture, where this enzyme usually acts, but is little warmer, to speed up metabolic rate)
2. Experimental Design:
In this experiment, we will be testing the optimal temperature for the functionality of the catechol oxidase enzyme. Our positive control is the sample taken at room temperature because we know that this will not denature the enzyme. We have no negative control because we do not know when this enzyme denatures.
PROCEDURE:
1. Label 4 test tubes 1–6.
2. Pipette 1 mL of catechol oxidase into each of the test tubes.
3. Add catechol into test tube 1. This is our control, and it will not be placed in the water baths because it is roughly 30 degrees Celsius already. This was tested in part D, and we have our result.
4. To make the water bath, heat or cool one water bath to 10, 20, and 40 degrees Celsius (do each one individually, for samples 1, 2, and 4). Heat with heat plate, and cool with ice.
5. Add the appropriate test tube to each bath, as shown in table 3.
| Test Tube Number |
Water Bath Temperature in Celsius |
| 1 |
0 |
| 2 |
10 |
| 3 |
25 (room temperature) |
| 4 |
40 |
| 5 |
60 |
| 6 |
80 |
| 7 |
100 |
6. Let it sit at the constant temperature required for 5 minutes.
7. Add 1 mL of catechol
8. Wait 5 minutes to observe if the temperature had any changes on the enzyme. Show resuts in table 4
0 no color change
1 little color change
2 more color change
3 dark color change
|
Tube
|
Result
|
Conclusion
|
|
1
|
2
|
|
|
2
|
2
|
|
|
3
|
2
|
|
| 4 |
3 |
|
| 5 |
3 |
|
| 6 |
0 |
|
| 7 |
0 |
|
Experiment #2: pH
1. Hypothesis:
Catechol oxidase will work best at a Ph of about 7, because that is the Ph where it is most neutral.
2. Experimental design:
Our positive control will be a Ph buffer of 7, because we know that the enzymes work under that condition . we dont really have a negative control, because we don't know whether or not the enzyme denatures.
1.) Set out test tubes, each with a different Ph buffer, including 2, 4, 6, 7, 8, 10, 12, 2 ml of each (label them 1-5)
2.) Put 1 ml of catechol, and 1 ml of potato extract in each tube.
3.) Shake tubes
4.) Record what happens, then after 5 minutes, we will record what happens again, in terms of color, on a scale of 1-5, as described above
|
Tube
|
Result
|
Conclusion
|
|
1
|
0
|
|
|
2
|
0
|
|
|
3
|
2
|
|
| 4 |
2 |
|
| 5 |
2 |
|
| 6 |
2 |
|
| 7 |
2 |
|
Experiment #3: Salt
1. Hypothesis:
The optimal salinity would be 0%, because extra solutes may interfere with the enzyme.
2. Experimental design: The control will be 20 ml of distilled water, because that has no salts. Our negative control will be 20 ml of the 10% saline solution, because we will expect that to inhibit enzyme activity.
1.) We need 5 test tubes, 1 for 20 ml distilled water, 1 for 15ml of distilled water and 5ml of 10% NaCl (for a 2.5% NaCl), then 1 for 10 ml distilled water and 10 ml of 10% NaCl (for 5% NaCl) then 5 ml distilled water, and 15ml NaCl ( for 7.5% NaCl) and finally on for 20 ml of 10% NaCl stock solution.
2.) We will put 1 ml of catechol and 1ml of potato extract in each test tube, let it sit, and record our observations, using the scale of 1-5 from above, every 5 minutes.
|
Tube
|
Result
|
Conclusion
|
|
1
|
|
|
|
2
|
|
|
|
3
|
|
|
| 4 |
|
|
| 5 |
|
|
Please embed your presentation below.
Comments (0)