A. Learning Objectives
In this lab, students will:
• analyze the effect of catechol oxidase on the production of benzoquinone.
• design and conduct experiments to study how physical conditions affect enzyme activity.
• plot data graphically.
B. Textbook Correlation
Please review Section 6.2 Enzymes and Ribozymes in Chapter 6: An Introduction to Energy, Enzymes, and Metabolism to assist in writing the intrroduction and researching the experiment.
C. Introduction
Please write a two paragraph introduction to enzymes;
Paragraph #1: Discuss the structure/function of enzymes. In your discussion, address the make-up of most enzymes, the role of the active site and its impact on specificity, and the idea behind the induced fit theory, Also discuss activation energy and how enzymes speed up chemical reactions by impacting the activation energy.
Enzymes are a class of catalysts. Catalysts are substances that speed up the rate of a chemical reaction without being consumed or permanently affected in the reaction. Enzymes are proteins and they are the most common types of catalysts in living cells. Reactions that are catalyzed with enzymes are millions of times faster than catalyzed reactions. Enzymes act in living cells by lowering the activation energy of reactions. When the reactants of a reaction try to come closer, there is a repulsion between them. This repulsion can only be overcome with an initial burst of energy, activation. However, this activation energy is prevents products from being formed rapidly. Enzymes function in lowering this activation energy, in order for the rapid production of products to occur. After lowering the activation energy enzymes also help the reactants reach a transition state, which is when original bonds in the reactants are stretched to their limit. This state must be reached in order for the reaction to continues. Enzymes strain reactants and bring reactants closer together, making it easier for the reactants to achieve a transition state.
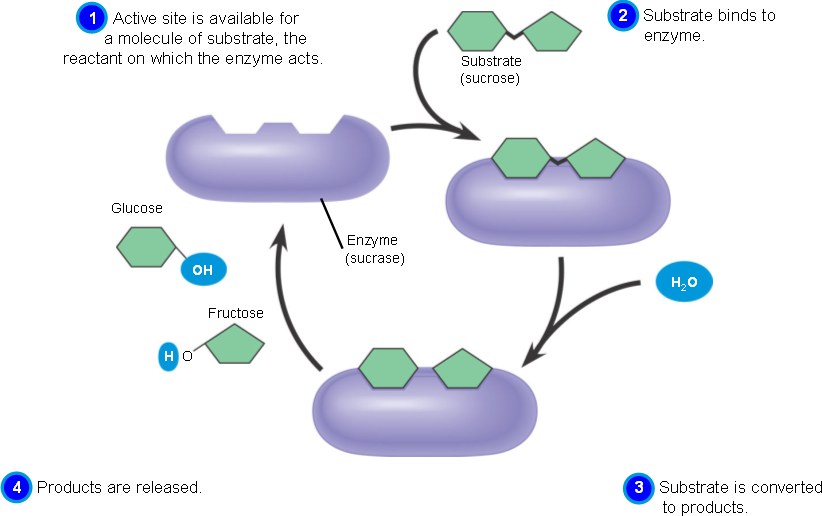
Enzymes are large proteins that bind to small reactants, called substrates. By binding to reactants, enzymes assist the reactants reach a transition state. Substrates bind to enzymes at their active site, which is the location in the enzymes where the chemical reaction will occur. Enzymes are very specific to which substrates they will bind to. When the enzyme and substrate are bound the resulting structure is called an enzyme-substrate complex. The enzyme-substrate complex exhibit a phenomenon called induced fit; this is when enzymes undergo conformational changes in order to perfectly fit the substrates that they are binding to. Enzymes also have allosertic sites. Noncompetitive inhibitors bind to the allosteric sites in order to stop enzymes activity. If a noncompetitive inhibitor does not stop the functioning of an enzyme, a catalytic cycle occurs. This cycle involves the binding of substrates to an enzyme, the formation of an enzyme-substrate complex , the formation of products (resulting in a free enzyme and products), and again the binding of substrates to an enzyme (and so on).

|

|
|
| This is the structure of a typical enzyme. This active site is the site where specific substrates bind to enzymes. The allosteric site is where noncompetitive inhibitors bind in order to stop the enzyme's function. |
This is an energy diagram of a chemical reaction that depicts the effects on an enzyme on activation energy. This shows that the activation energy of a chemical reaction is much lower when an enzyme acts on it, than when an enzyme does not. The rate of a chemical energy is higher when an enzyme lowers the activation energy of the reaction. |
This figure demonstrates the catalytic cycle of an enzyme. Substrates attach to an enzyme at the active site, an enzyme-substrate complex is formed, the product is formed (resulting in a free enzyme and products), and again substrates attach to an enzyme (and so on). This cycle continues until a noncompetitive inhibitor attaches to the allosteric site of an enzyme. |
Paragraph #2: Discuss how enzyme activity is regulated in a cell. Include in the discussion the idea of enzyme saturation, how saturation is overcome, the physical requirements for optimal enzyme activity, and the role of inhibitors (both competitive and non-competitive). When discussing inhibitors, include the idea behind allosteric regulation.
Enzyme activity in cells is regulated not only by enzyme specificity as discussed above, but also by several forms of inhibition and by substrate concentration. When an enzyme recognizes the specific substrate that fits its active site, the enzyme will react with it and convert it to a product normally. The higher the concentration is of the substrate, the faster an enzyme will generate the products. This can be seen in the first image below on the graph sowing substrate concentration versus velocity of products/second produced by the enzyme. At a certain point of concentration, it is clear that the reaction rate plateaus. This is because the active sites of the enzymes are all occupied with substrates. In other words, the enzymes are saturated with substrate and cannot catalyze reactions at any faster of a rate. The point at which the reaction rate is at it's highest and will not increase regardless of substrate concentration, is called Vmax. The Km point is the substrate concentration at which Vmax is at 1/2. This is significant because it is considered a constant for which enzymes can be compared by. An enzyme with a higher Km needs a higher substrate concentration to reach certain velocities whereas an enzyme with a lower Km does not need as high of a substrate concentration.
There are several conditions under which enzymes perform at their optimal levels. Dramatic temperature changes can greatly affect an enzymes performance. If the temperature is too high, the kinetic motion of the amino acids forming an enzyme will disrupt the three-dimensional shape and stability of the enzyme, which is known as denaturation. If the temperature is too low, the molecular motion of the environment as a whole is so low that the frequency of collision between enzymes and substrates decreases. Each enzyme has its own optimal temperatures that it functions best at. The other factor that affects enzyme activity is pH. Because the amino acids of an enzyme are charged, the pH of the environment is crucial to allow for the proper formation of the protein structure. If it is too acidic, meaning a higher concentration of H+ ions, then negatively charged group such as carboxyl (-COO-) will be pushed towards becoming neutral (-COOH). This stops the enzyme from being able to form ionic bonds that are vital to its three-dimensional shape and it denatures. Again, there are unique optimal pH's for different enzymes.
A major factor for enzyme regulation is the role of inhibitors. There are two key types that are reversible: competitive and noncompetitive inhibition. During competitive inhibition, a molecule will bind to the active site of an enzyme. It is not a covalent bond but it stops the substrate from being able to bind and thus stops the enzyme from being able to carry out its reactions. The inhibiting molecule is generally similar in structure to the normal substrate for that enzyme and that is why it can penetrate the high specificity of enzymes. In noncompetitive inhibition, a molecule will bind noncovalently to an allosteric site of the enzyme. The allosteric site is a location outside the active site. This is often used in feedback inhibition, a process by which the product of the metabolic pathway acts as a noncompetitive inhibitor. This allows a cell to regulate the production of a certain product because when the product accumulates it will bind to the allosteric site of one of the enzymes in order to stop the metabolic pathway from functioning properly and thus stopping production of the final product. This can be seen in the third animation below.
In today’s exercise you will first observe the actions of the enzyme catechol oxidase. After this exercise you will be ready to design two experiments on your own to test the physical requirements for optimal enzyme activity.
D. Catechol Oxidase Activity
In today’s exercise the enzyme you will use is catechol oxidase. In plants this copper-containing enzyme creates brown pigment when exposed to air (specifically oxygen), and it is the reason fruits turn brown after they are sliced. The brown color is due to the production of the product benzoquinone, a substance that is toxic to food-spoiling bacteria. When the peel is damaged, oxygen can then react with the catechol, protecting the fruit.
In this experiment, we will test catechol oxidase activity. The enzyme is extracted from potatoes using a blender and is referred to as potato extract in the subsequent experiments.
Experimental Procedure:
1. Label 3 test tubes 1–3.
2. Pipette the amount of catechol and water into the appropriate test tube as outlined in Table 1. Do not add the catechol oxidase to all tubes until just before starting the incubation in step 3.
|
Tube
|
mL of Catechol
|
mL of Water
|
mL of Catechol Oxidase
|
|
1
|
1
|
0
|
1 mL (20 drops)
|
|
2
|
0
|
1
|
1 mL (20 drops)
|
|
3
|
1
|
1
|
0 mL (0 drops)
|
3. Place the test tubes in the 37⁰C water bath for 10 minutes.
4. Record your results in the table above. Use the following scale:
0 no color change
1 little color change
2 more color change
3 dark color change
|
Tube
|
Result
|
Conclusion
|
|
1
|
|
|
|
2
|
|
|
|
3
|
|
|
Questions
1. Which tube is the negative control? Which tube is your positive control?
2. What would it mean if tube 2 turned brown?
E. Design an Experiment to Study Enzyme Activity Under Different Physical Conditions.
Protein activity is highly dependent on its three-dimensional structure. Conditions that cause a protein to denature (unfold) results in the loss of protein activity. Environmental deviations from optimal cause an enzyme to lose activity. Question: What is the optimal temperature for catechol oxidase activity? What is the ideal pH for catechol oxidase activity? What is the optimal salinity for catechol oxidase activity? Use the experiment from section D as a template. Remember to include positive and negative controls when applicable. Make sure you take photographic images of your results and a video of your procedure explaining how you designed the experiment.
Materials Provided:
- test tubes
- plastic pipettes
- catechol
- potato extract
- water baths (3) and hot plate
- ice
- thermometers
- phosphate buffers ranging from pH of 2 to 12 (actual buffers available (in pH units): 2, 4, 6, 7, 8, 10, 12)
- distilled water
- 10% NaCl stock solution
Experiment #1: Temperature
1. Hypothesis: If the temperature of the solution is not 37 degrees Celsius, the rate of reactions will be inhibited
2. Experimental Design:
For all tests, do not add cathecol until just before incubation
| Test Tube |
Cathechol Oxidase |
Cathechol |
Temperature |
| 1 (40) |
1 mL |
1 mL |
40 degree Celsius |
| 2 (60) |
1 mL |
1 mL |
60 degrees Celsius |
| 3 (80) |
1 mL |
1 mL |
80 degrees Celsius |
| 4 (room temperature) |
1 mL |
1 mL |
Room Temperature |
| 5 (5 degrees Celsius) |
1 mL |
1 mL |
5 degrees Celsius (Ice Bath) |
| 6 (100 degrees Celsius) |
1 mL |
1 ml |
100 degrees Celsius |
1. Fill Test tubes with the above amounts of substance
2. Place each test tube in a water bath of the given temperature for 10 minutes
3. Observe color change
Experiment #2: pH
1. Hypothesis: If the pH is greater than or less than 7, enzyme activity will be inhibited.
2. Experimental design:
For all tests, do not add cathechol until right before adding to water
| Yest Tube |
Cathechol Oxidase |
Cathechol |
pH buffer |
| 1 (7) |
1 mL |
1 mL |
7 (1 mL) |
| 2 (2) |
1 mL |
0 mL |
2 (1 mL) |
| 3 (4) |
1 mL |
1 mL |
4 (1 mL) |
| 4 (10) |
1 mL |
1 mL |
10 (1 mL) |
| 5 (12) |
1 mL |
1 mL |
12 (1 mL) |
1. Fill test tubes with required amount of substance
2. Place test tubes in a water bath of 37 degrees Celsius for 10 minutes
3. Observe color change
Experiment #3: Salt
1. Hypothesis: If the concentration of salt is outside of its optimum concentration of 0.9%, then enzyme activity will be inhibited.
2. Experimental design:
Do not add cathechol to test tubes until right before placing in water
| Test tube |
Water |
Cathechol Oxidase |
Cathechol |
NaCl |
| 1 (positve) |
34 drops |
10 drops |
10 drops |
6 drops |
| 2 (negative) |
44 drops |
10 drops |
0 drops |
6 drops |
| 3 (5%) |
10 drops |
10 drops |
10 drops |
30 drops |
| 4 (0%) |
40 drops |
10 drops |
10 drops |
0 drops |
1. Fill test tubes with required amount of substance
2. Place test tubes is water bath of 37 degrees Fahrenheit for 10 minutes.
3. Observe color change.
Please embed your presentation below.
Comments (0)